
BH3 Lewis Structure (Boron Trihydride) YouTube
70 More Lewis Dot Structures. Boron will not form an octet, which is why this does not occur extensively in nature. Borane is only formed as a gas and readily oxidizes in the air, sometimes violently.

How to draw BBr3 Lewis Structure? 4
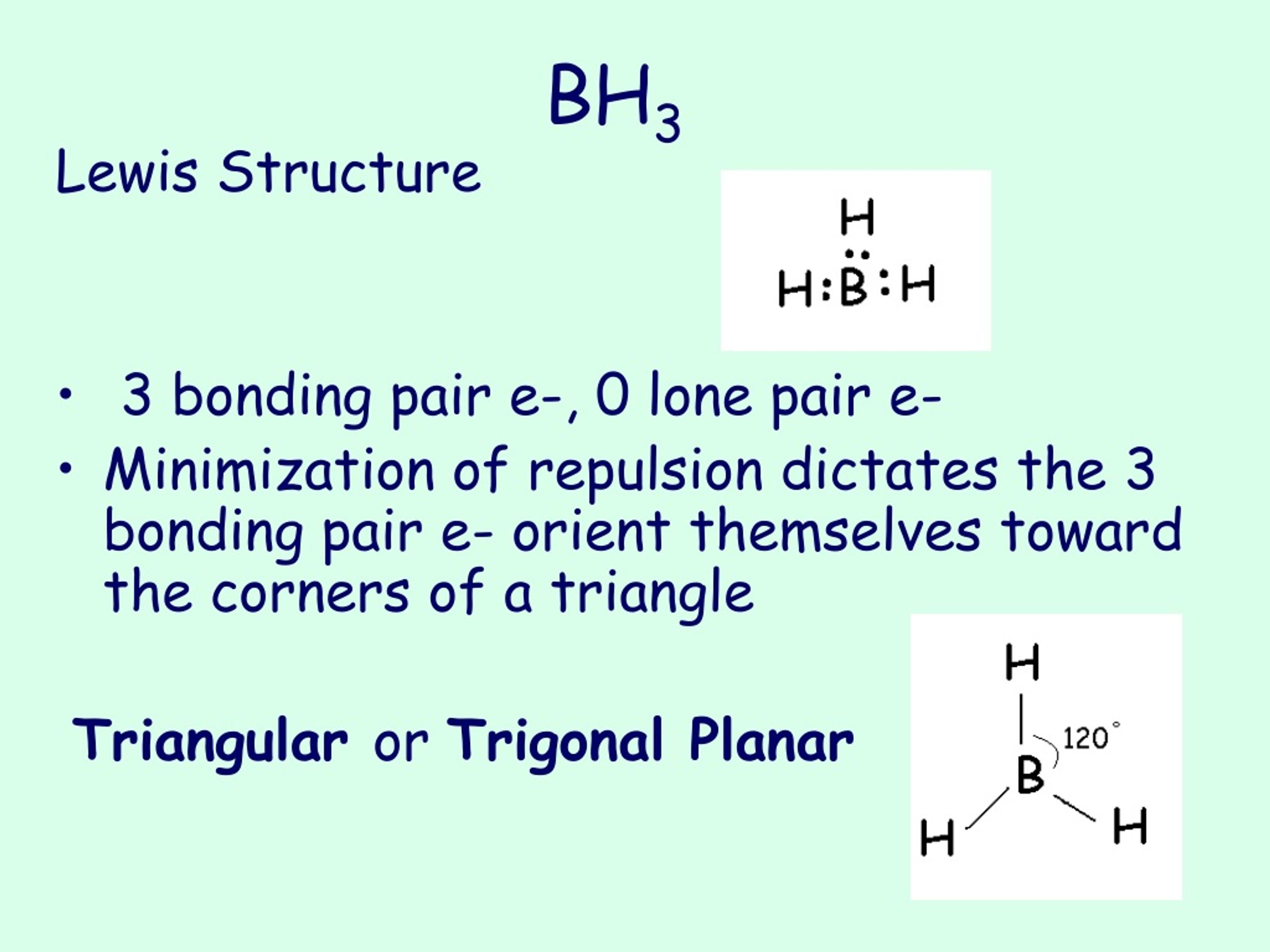
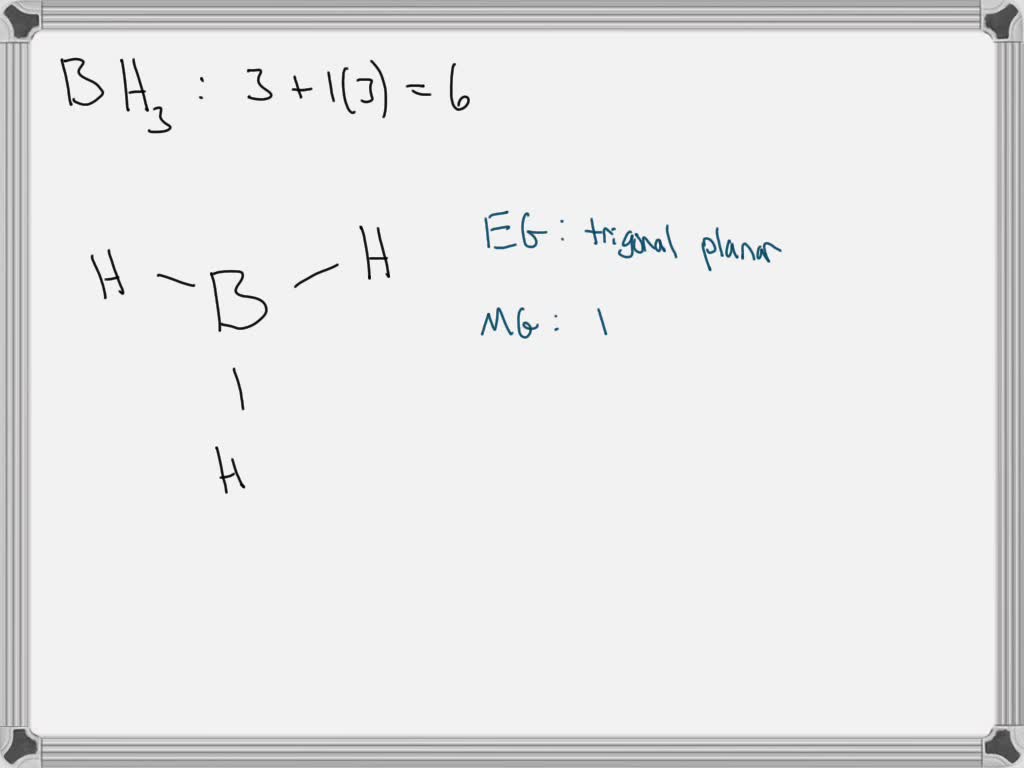
Borane (BH 3) is a lewis acid and there are one boron atom and three hydrogen atoms in borane molecule. Each hydrogen atom has connected with boron through a single bond in the lewis structure of borane (BH3). There are only three bonds around boron atom and no lone pairs on boron atom.

BH3 molecular geometry, lewis structure, bond angle, hybridization
Thus, boron commonly forms three bonds, BH 3 , with a total of six electrons in the outermost shell. This also results in some anomalous properties for boron compounds because they are kind of "short of electrons". It should be thus noted that covalent bonding between non-metals can occur to form compounds with less than an octet on each atom.

Lewis Dot Structure Of Nh3
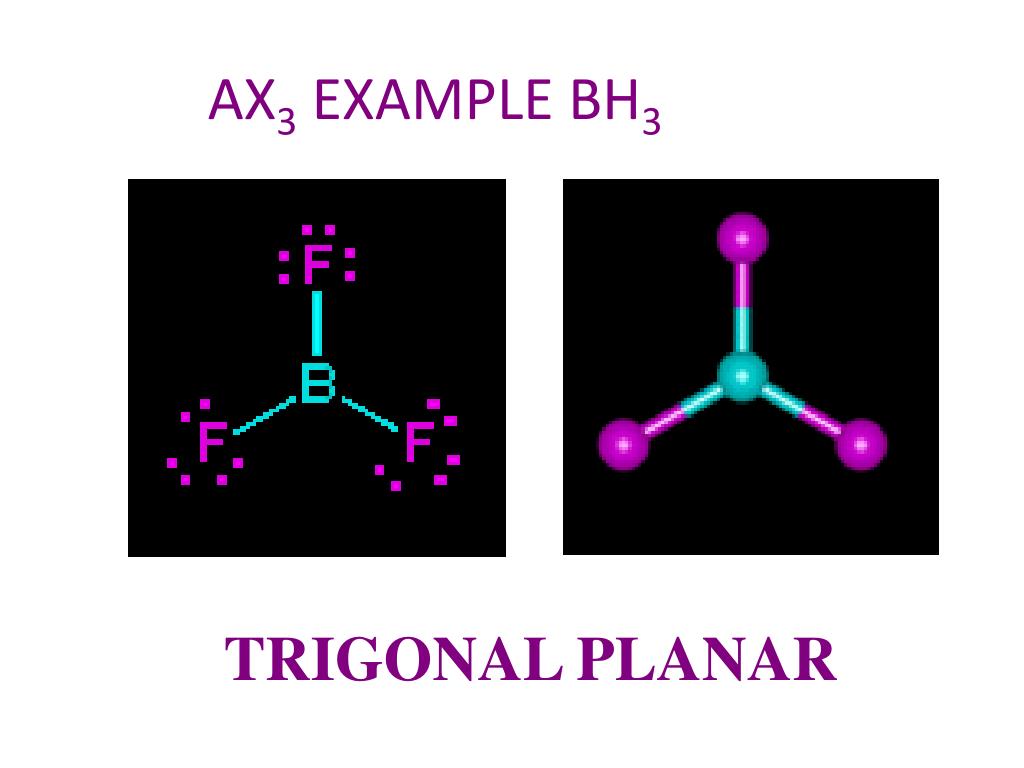
BH 3 is a trigonal planar molecule with D 3h symmetry. The experimentally determined B-H bond length is 119 pm. [5] In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: [6] BX 3 +BH 4− → HBX 3− + (BH 3) (X=F, Cl, Br, I)

BF3 Lewis Structure, Molecular Geometry, and Hybridization
The molecule adopts a structure similar to that of ethane, with which it is isoelectronic. The B−N distance is 1.58 (2) Å. The B−H and N−H distances are 1.15 and 0.96 Å, respectively. Its similarity to ethane is tenuous since ammonia borane is a solid and ethane is a gas: their melting points differing by 284 °C.
PPT Molecular Geometry PowerPoint Presentation, free download ID343710
Drawing the Lewis Structure for BH 3. Viewing Notes: The BH 3 Lewis structure is similar to BF 3, BCl 3 and BBr 3 since F, Cl, and Br are all in Group 7 and have 7 valence electrons.. Let's do the Lewis structure for BH3. On the periodic table, Boron's in group 3. It has 3 valence electrons. Hydrogen's in group 1, but we have 3 Hydrogens; so.
Lewis Electron Dot Structure For Boron
Lewis structures of \(\ce{BH3}\) and \(\ce{BF3}\) were described in Exercise 3.1.2, and are drawn again below for convenience.. In its pure form, the compound actually exists as a dimeric gas with a molecular unit of B 2 H 6 (try drawing a valid Lewis structure for that!). Its unexpected structure includes two H's that bridge the two boron.

Chemfig How Can I Draw A Lewis Structure Tex Latex Stack Exchange Hot
In the BH 3 Lewis structure, there are three single bonds around the boron atom, with three hydrogen atoms attached to it, and none of the atoms has a lone pair. BH3 Lewis Structure - How to Draw the Lewis Structure for BH3 Watch on Contents Steps #1 First draw a rough sketch #2 Mark lone pairs on the atoms External links Steps

Covalent Lewis Dot Structures Worksheet Worksheets For Kindergarten
Boron trihydride has a stable sextet. It is extremely reactive - even reacting with itself if there is nothing else.0:00 Lewis structures1:49 Shape and angl.

Incredible Is Bh3 Polar Or Nonpolar References
Check me out: http://www.chemistnate.com
Lewis dot structure and hybridisation of BH3 Borane lewis structure
Steps of drawing BH3 lewis structure Step 1: Find the total valence electrons in BH3 molecule In order to find the total valence electrons in BH3 molecule, first of all you should know the valence electrons present in boron atom as well as hydrogen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Bh3 ün lewis nokta gosteriminde Bor un elektronlari toplami bağ sonunda
Lewis structure of BH3 contains three single bonds between the Boron (B) atom and each Hydrogen (H) atom. The Boron atom (B) is at the center and it is surrounded by 3 Hydrogen atoms (H). Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me.
PPT Lewis Structures PowerPoint Presentation, free download ID5585056
The Lewis base donates an electron pair to form a covalent bond with the Lewis acid (Fig. 4.1.2). A covalent bond formed in a Lewis acid-base reaction is usually called a dative bond because both electrons in the covalent bond come from a single partner. In a "conventional" covalent bond both partners contribute one electron to the covalent bond.

Lewis structure/sharing of electrons of bh3 Brainly.ph
Home Bookshelves Organic Chemistry Organic Chemistry I (Liu) 1: Basic Concepts in Chemical Bonding and Organic Molecules

The structure of BH3 is Chemistry Questions
A step-by-step explanation of how to draw the BH3 Lewis Dot Structure (Boron Trihydride).There are only 6 valence electrons in the Lewis structure for BH3.Th.
SOLVED Question 27 For the molecule given bclow BH3 Draw the Lewis
Lewis Dot Structure of BH3 (Boron Hydride) - YouTube 0:00 / 1:11 Lewis Dot Structure of BH3 (Boron Hydride) kentchemistry.com 24.8K subscribers 58K views 11 years ago I quickly take you.