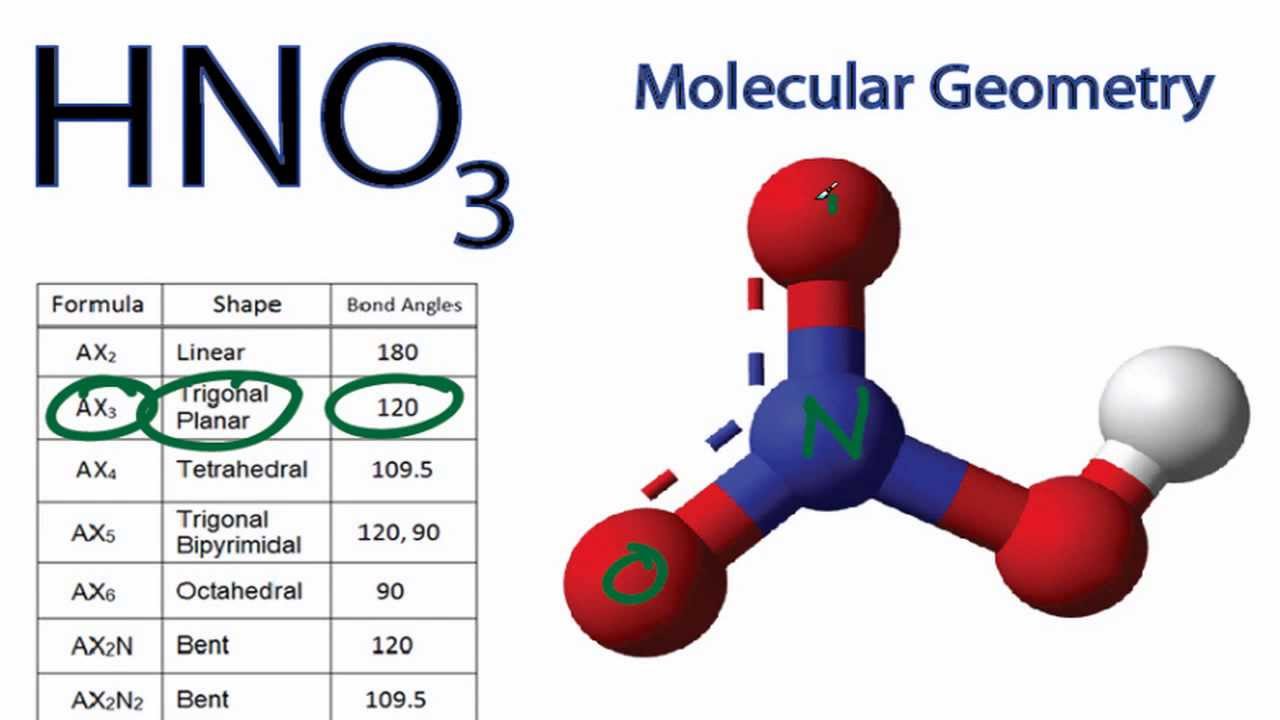
HNO3 Molecular Geometry / Shape and Bond Angles YouTube
1. Count the total valence electrons in HNO3 The very first step while drawing the Lewis structure of HNO 3 is to calculate the total valence electrons present in its concerned elemental atoms. In HNO 3, there are atoms from three different elements of the Periodic Table.

SOLVED LeA ouuttTaddb Ham Prelaboratory Assignment Molecular Geometry
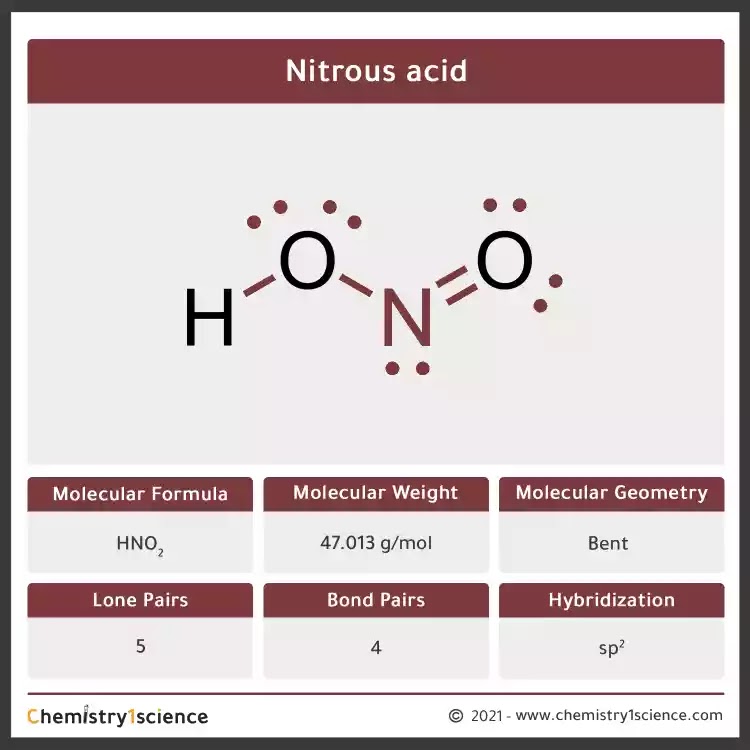
Step 1. We begin by counting the total number of valence electrons of the molecule. Looking at HNO2, we see that H has 1 valence electron, N has 5 valence electrons and O has 6 valence electrons and there are two atoms of O, so 6×2 = 12 valence electrons.

HNO2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
By using formula π = 6n + 2 − V π = 6 n + 2 − V where π π is number of π π electrons, n n is number of atoms excluding all hydrogen atom, and V V is total valence, the π π is 2 2. It means that there would be one double bond in the structure of HNOX3 H N O X 3 and the remaining bonds other than π π bond are σ σ bond.

Nitric acid 3D Model HNO3 free 3D model CGTrader
There are two resonance structures HNO3 (Nitric Acid). We start with a valid Lewis structure and then follow these general rules. For the HNO3 resonance stru.
[Solved] Draw the Lewis structure for nitric acid (HNO₃). How many
Commercially available nitric acid is an azeotrope with water at a concentration of 68% HNO3. This solution has a boiling temperature of 120.5 °C (249 °F) at 1 atm. It is known as "concentrated nitric acid". The azeotrope of nitric acid and water is a colourless liquid at room temperature.

HNO3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
323 2 3 8 The left one satisfies 8-electron rule (something like that.) - user26143 Dec 2, 2013 at 17:56 Yeah but let's take a look at another example: H3PO4 Although the octet formation is not fulfilled, the structure with no formal charges is preferred. Here is the comparison: i.imgur.com/XLpgIjn.png - Zafer Cesur Dec 2, 2013 at 18:03
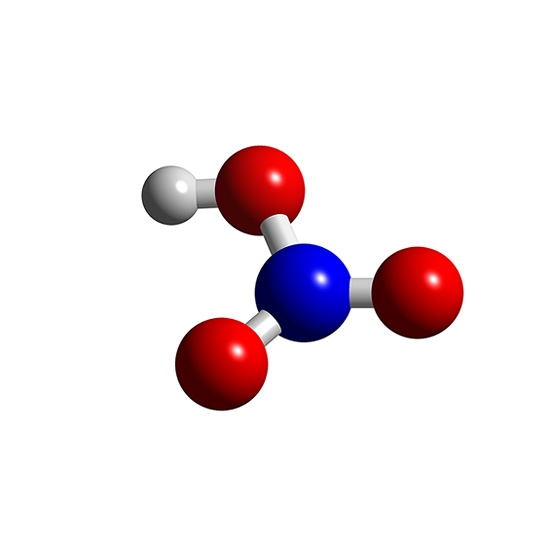
Ácido Nítrico Compostos Químicos InfoEscola
The Lewis structure of HNO₃ shows that it is a resonance hybrid of two structures. The N atom has steric number SN = 3. The electron geometry is trigonal planar. The N atom is sp² hybridized. The O bonded to H has SN = 4 (two bonding pairs and two lone pairs). The electron geometry is tetrahedral. This O atom is sp³ hybridized.

HNO3 Polar or Nonpolar (Nitric Acid) YouTube
Drawing the Lewis Structure for HNO 3. The HNO 3 Lewis structure is easier to think of if you consider it NO 3 with an H bonded to one of the oxygen atoms. In HNO 3 Lewis structure Nitrogen (N) is the least electronegative atom and goes in the center of the Lewis structure. Check the formal charges to be sure that each atom has a formal charge.

Рынок азотной кислоты в Украине
For the very first time, the molecular geometry of HNO has been experimentally determined by Dalby, based on the constants obtained from the rotational analysis (Dalby, 1958). For the HNO singlet ground state r N-O = 1.212 Å, r N-H = 1.063 Å, and θ H-N-O = 108.6°.
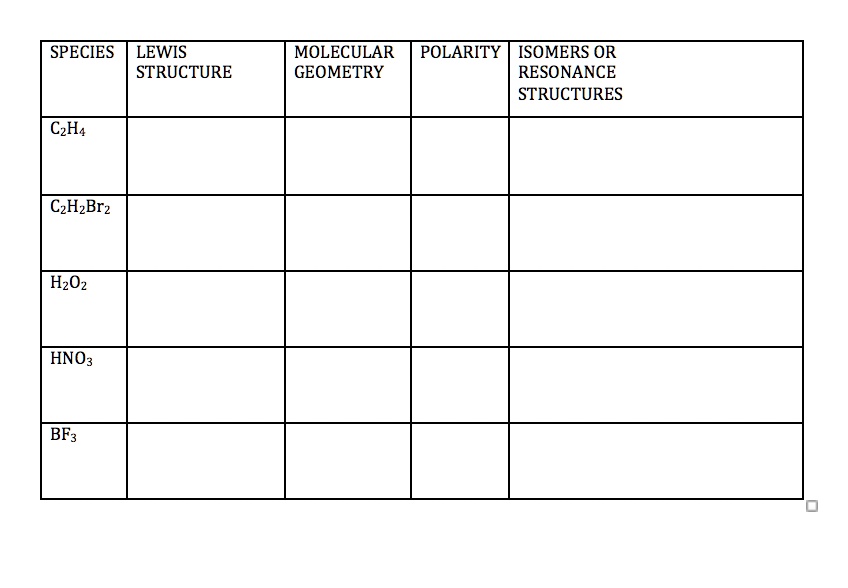
SOLVED SPECIES LEWIS STRUCTURE MOLECULAR POLARITY ISOMERS OR GEOMETRY
HNO3 is an interesting chemical to study. In the next sections, we will discuss the Lewis structure, molecular geometry, and hybridization of this acid. Contents show Lewis Structure of Nitric Acid (HNO3) Nitric acid has the molecular formula of HNO3, which means it has one hydrogen atom forming bonds with nitrate ion.
Hno Lewis Structure Shape
HNO3 Lewis Structure - How to Draw the Lewis Structure for HNO3 10 years ago Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry 777K views 6 years ago Lewis.
Hno3 Molecular Geometry Vector Icon Stock Vector Illustration of
The NITRIC ACID molecule contains a total of 4 bond (s). There are 3 non-H bond (s), 2 multiple bond (s), 2 double bond (s), and 1 hydroxyl group (s). Images of the chemical structure of NITRIC ACID are given below: The 2D chemical structure image of NITRIC ACID is also called skeletal formula, which is the standard notation for organic molecules.

explain the structureof hno3 Chemistry Chemical bonding and
Draw the Lewis Structure of HNO3 Molecular Geometry? Ideal Bond Angles? Are there Polar Bonds Present (Indicate on Lewis do structure Is there an Overall Dipole? Octet rule violator and how? BUY. Chemistry: An Atoms First Approach. 2nd Edition. ISBN: 9781305079243.

HNO3 Lewis structure, molecular geometry, hybridization, polar or nonpolar
Chemistry Chemistry questions and answers For molecule nitric acid HNO3 1) write its molecular geometry; kind of hybridization does N take? 2) How many σ and π bonds?; 3) what This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
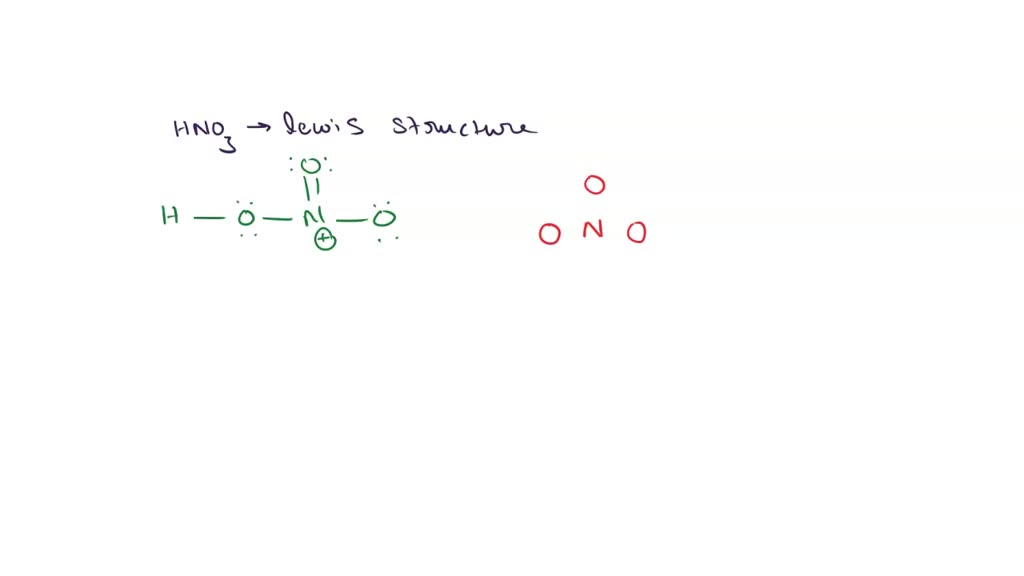
SOLVED Draw the Lewis structure for HONO2 (HNO3) (including double
Molecular Weight 63.013 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2004-09-16 Modify: 2024-01-06 Description Nitric acid, red fuming appears as a pale yellow to reddish brown liquid generating red-brown fumes and having a suffocating odor. Very toxic by inhalation. Corrosive to metals or tissue.

HNO3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Structural Formula. HNO 3. nitric acid